Injection Guides
View

Welcome to the step-by-step guide of how to take your Fostimon injections. This video will explain how to prepare, inject and dispose of your treatment. Please refer to your clinic for the specific dose prescribed to you. Your dose may be adjusted over the course of your treatment. Please refer to your healthcare professional for any questions.
View

Download

Download

Explore our interactive FAQ section for comprehensive Fostimon injection guidance. This area is designed to provide you with concise yet comprehensive answers regarding the proper usage and administration of Fostimon injections.
Please read your patient information leaflet for full information.
Urofollitropin is a highly purified human follicle stimulating hormone, belonging to a group of medicines called gonadotropins. This medicinal product must be used under the supervision of your doctor.
You and your partner’s fertility will be evaluated before your treatment is started. DO NOT USE FOSTIMON if you have any of the following:
This medicine should not be used if you have an early menopause, a malformation of the sexual organs or certain tumours of the womb that would make a normal pregnancy impossible.
TAKE SPECIAL CARE WITH FOSTIMON
Although no allergic reactions to Fostimon have yet been reported, you should tell your doctor if you have an allergic reaction to similar medicines.
This treatment increases your risk of developing a condition known as ovarian hyperstimulation syndrome (OHSS) (see Possible side effects). If ovarian hyperstimulation occurs then your treatment will be stopped and pregnancy will be avoided. The first signs of ovarian hyperstimulation are pain in the lower abdominal region as well as nausea (feeling sick), vomiting and weight gain. If these symptoms occur you should be examined by your doctor as soon as possible. In serious, but rare cases, the ovaries can become enlarged and fluid can build up in the abdomen or chest. The drug used to bring about the final release of mature eggs (containing human chorionic gonadotropin-hCG) can increase the likelihood of OHSS. It is therefore not advisable to use hCG in cases where OHSS is developing and you should not have sexual intercourse even if using a barrier methods of contraception for at least 4 days.
It should be noted that women with fertility problems have a higher rate of miscarriages than the normal population.
In patients having treatment to help ovulation, the occurrence of multiple pregnancies and births is increased compared to natural conception. However, this risk can be minimised by using the recommended dose.
There is a slightly increased risk of extra-uterine pregnancy (an ectopic pregnancy) in women with damaged fallopian tubes.
Multiple pregnancies and characteristics of the parents undergoing fertility treatments (e.g. maternal age, sperm characteristics) may be associated with an increased risk of birth defects.
Treatment with Fostimon, just as pregnancy itself, may increase the chance of having thrombosis. Thrombosis is the formation of a blood clot in a blood vessel, most often in the veins of the legs or the lungs.
Please discuss this with your doctor, before starting treatment, especially:
This medicine is prepared from human urine. The risk of passing on an organism that could cause an infection or disease cannot be definitely excluded; however, this is limited by steps in the manufacturing process to remove viruses, especially HIV, Herpes virus and Papillomavirus.
No cases of viral contamination have been reported.
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Fostimon should not be used if you are pregnant or breast-feeding.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
Always take this medicine exactly as yourdoctor has told you. Check with your doctor if you are not sure.
If you are having periods, the treatment should start within 7 days of the start of your period (the first 7 days of the menstrual cycle).
You will be given 1 injection per day under your skin (subcutaneous).
The usual starting dose is 75 to 150 IU of FSH (Fostimon) every day. This dose may be increased, if necessary, by 37.5 to 75 IU at 7 or preferably 14-day intervals, to get the right response.
The maximum daily dose of FSH is usually not higher than 225 IU.
If your doctor cannot see a response after 4 weeks of treatment, that treatment cycle will be stopped. For the following cycle, your doctor will prescribe a higher starting dose.
When you get a good response (satisfying follicle growth) you will be given a single injection of another medicine (hCG), which is used to bring about the final maturing of the follicle and release of eggs. This will be given 24 to 48 hours after the last Fostimon injection. You should have sexual intercourse the day hCG is given and again on the following day.
If you have too great a response, treatment will be stopped and hCG will not be given (see Possible side effects). For the following cycle, your doctor will prescribe a lower starting dose.
Women undergoing ovarian stimulation for multiple follicular development prior to in vitro fertilisation or other assisted reproductive techniques:
The treatment should start 2 or 3 days after the start of your periods (the first 2 or 3 days of the menstrual cycle).
You will be given 1 injection per day by the subcutaneous route.
The usual starting dose for superovulation is 150 to 225 IU of Fostimon every day. Treatment is continued, with the dose adjusted according to your response, until you are achieving adequate follicular development. This is usually achieved on average by the 10th day of treatment (range 5 to 20 days) and is measured by taking blood samples and/or ultrasound examinations.
The maximum dosage is in general of 450 IU/ day.
Once adequate follicular development is achieved you will be given a single injection of a medicine used to bring about final maturing of the follicle; this medicine contains up to 10,000 IU human chorionic gonadotropin (hCG). It will be given 24 to 48 hours after the last Fostimon injection.
Oocytes will be punctured about 35 hours later.
Fostimon will be given approximately 2 weeks after the start of this treatment. Both treatments are continued until adequate follicular development is achieved. Fostimon will be given as 1 injection per day by the subcutaneous route. For example, following two weeks of treatment with an agonist of GnRH, 150 to 225 IU of Fostimon will be given for the first 7 days. The dose will then be adjusted according to the ovarian response.
Fostimon is given by injection either under your skin (by the subcutaneous route) or into a muscle (by the intramuscular route).
Each vial should be used only once and the injection should be used as soon as it is prepared.
After suitable advice and training your doctor may ask you to inject Fostimon yourself.
For the first time, your doctor must:
Presentations other than ampoules should be considered for self-administration by patients.
Before injecting Fostimon yourself, read the following instructions carefully.
The solution must be prepared just before injection. One vial is for single use only.
The medicinal product must be reconstituted under aseptic conditions.
Fostimon must only be reconstituted with the solvent provided in the package.
Prepare a clean surface and wash your hands before the solution is reconstituted. It is important that your hands and the items you use are as clean as possible.
Set out all the following items on the clean surface:
Like all medicines, Fostimon can cause side effects, although not everybody gets them. The following side effect is important and will require immediate action if you experience it.
You should stop taking Fostimon and see your doctor immediately if the following occurs:
Common, affects 1 to 10 users in 100:
The following side-effects have also been reported:
Common, affects 1 to 10 users in 100:
Uncommon, affects 1 to 10 users in 1,000:
Redness, pain and bruising at the injection site may occur (frequency not stated).
See section 2 for additional information on risk of blood clots, ectopic pregnancy, multiple pregnancy and miscarriage.
If any of the side effects gets serious, or if you notice any side effects not mentioned in this leaflet, please tell your doctor or pharmacist.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed here.
You can also report side effects directly via the Yellow Card Scheme www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Keep the vial and the ampoule of solvent in the outer carton in order to protect from light.
Do not use this medicine after the expiry date which is stated on the outer carton, the vial, and the ampoule of solvent. The expiry date refers to the last day of the month.
Use immediately after reconstitution.
Do not use Fostimon if you notice the solution does not look clear. After reconstitution the solution must be clear and colourless.
Do not throw away any medicines via wastewater. Ask your pharmacist how to safely dispose of medicines you no longer use. These measures will help protect the environment.
The active substance is Urofollitropin.
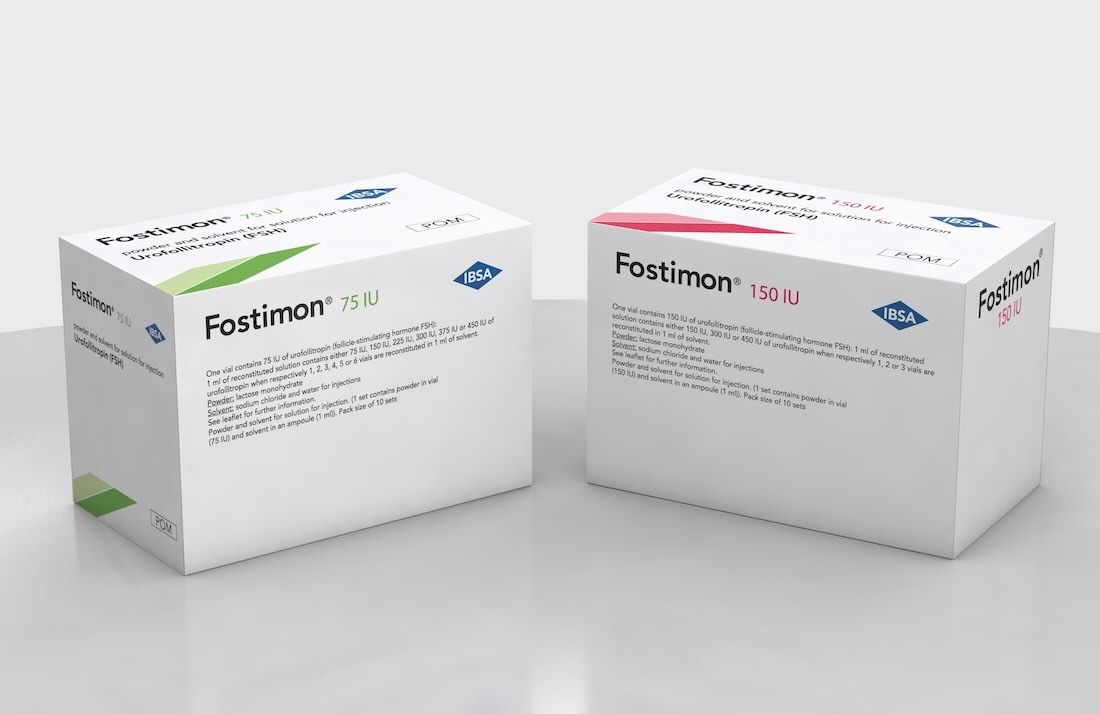
One vial contains 75 IU of urofollitropin (follicle-stimulating hormone FSH): 1 ml of reconstituted solution contains either 75 IU, 150 IU, 225 IU, 300 IU, 375 IU or 450 IU of urofollitropin when respectively 1, 2, 3, 4, 5 or 6 vials are reconstituted in 1 ml of solvent.
One vial contains 150 IU of urofollitropin (follicle-stimulating hormone FSH): 1 ml of reconstituted solution contains either 150 IU, 300 IU or 450 IU of urofollitropin when respectively 1, 2, or 3 vials are reconstituted in 1 ml of solvent.
The specific in vivo activity is equal or superior to 5000 IU of FSH per mg of protein.
For the powder: lactose monohydrate.
For the solvent: sodium chloride and water for injections
Fostimon is presented as a powder and solvent for solution for injection. 1 set contains powder in vial (75 IU or 150 IU) and solvent in an ampoule (1ml) - Pack size of 1, 5 or 10 sets.
The powder is a white to off-white caked mass and the solvent is clear and colourless.
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italy
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italy
IBSA Pharma Limited
Units 4-6 Colonial Business Park, Colonial Way, Watford WD24 4PR, UK
This medicinal product is authorized in the Member States of the EEA under the following names: (The strength and pharmaceutical form are identical in all countries, only the trade name changes)
Austria: Fostimon
Belgium: Fostimon
Cyprus: Fostimon
Denmark: Fostimon
Finland: Fostimon
France: Fostimon
Luxembourg: Fostimon
Ireland: Fostimon
The Netherlands: Fostimon
Norway: Fostimon
Spain: Fostipur
Sweden: Fostimon
United Kingdom: Fostimon
If you get any side-effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side-effects directly via the Yellow Card Scheme at yellowcard.mhra.gov.uk.
By reporting side-effects, you can help provide more information on the safety of this medicine.
To report a side effect or product complaint to IBSA Pharma please contact IBSA Pharma Ltd on 01923 233466 and medicalinformation.uk@ibsagroup.com.